Cellular reprogramming
Cellular reprogramming has highlighted the plasticity of mature cells, providing new technologies to generate any cell type of choice. Through enforced expression of cell type-specific transcription factors, with ability to bind DNA and regulate gene expression, it is possible to reprogram somatic cells to pluripotency or directly into other cell fates of choice.
Direct cell reprogramming has proven successful to convert skin cells into other cell types, including blood stem and progenitor cells, neurons, heart and liver cells, using transcription factors specifying target-cell identity. Until recently this technology has been mainly focused in the generation of functional autologous cells for regenerative medicine. At Asgard Therapeutics, we pioneered the development of reprogramming strategies to generate functional immune cell types able to set-in-motion immune responses.
Cancer immunotherapy
Cancer immunotherapy employs components of the immune system to fight cancer. In contrast to traditional radiotherapy and chemotherapy, cancer immunotherapies have shown a remarkable benefit by providing long-term responses, increasing patient survival several years after treatment.
These therapies have positioned themselves as the first-line treatment for several indications and the number of patients eligible for immunotherapy keeps increasing. Nevertheless, effective treatments are still not available for several cancer indications and the majority of treated patients do not respond or develop acquired resistance to these therapies. This limited effectiveness is due to the development of tumor evasion mechanisms, including immunosuppression of the tumor microenviroment (TME), and downregulation of antigen-presentation by tumor cells that bypass immune detection and tumor clearance.
Dendritic cells
Dendritic cells (DCs) are an heterogenous family of immune cells at the interface between innate and adaptive immune systems. Among the mononuclear phagocytes, DCs are known as professional antigen-presenting cells due to their unique ability to sense, process and present foreign antigens to T cells, initiating potent antigen-specific immune responses. This capacity further relies on the ability of DCs to secrete immunomodulatory cytokines and chemokines, together with the expression of high levels of major histocompatibility complexes and co-stimulatory molecules at the cell surface.
Recent studies have highlighted the critical role of a subset of DCs, called conventional type 1 DC (cDC1), in orchestrating cancer immunity and driving clinical response to emerging cancer immunotherapies, including checkpoint blockage and adoptive T cell therapy. However, cDC1s are found in very limiting numbers in vivo and are challenging to produce ex vivo. The in vivo generation of functional DCs within tumors opens the opportunity to explore the unique features of DCs and induce anti-cancer immunity by allowing the generation of the right cell type in the right location.
This is our
lead program
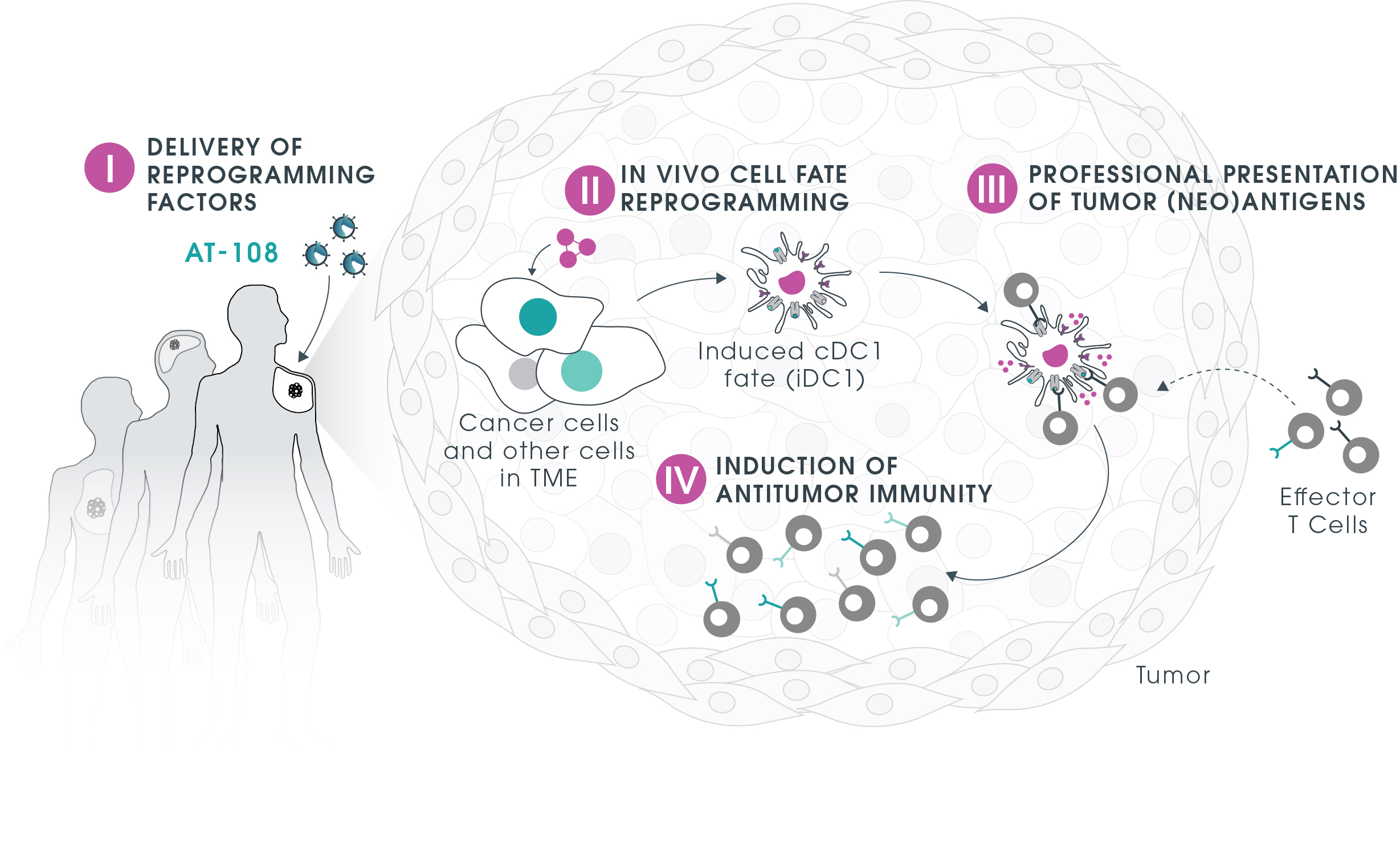
Our lead program AT-108 is pioneering an off-the-shelf personalized treatment applicable to a spectrum of cancer indications. This seemingly paradoxical approach takes advantage of the Trojan Horse concept (TrojanDC) that can be administered to different patients inducing presentation of the tumor(neo) antigens and activating personalized anti-tumor responses.
AT-108 mechanism of action
OFF-THE-SHELF
- One-size-fits-all approach
- Platform potential
- Straightforward manufacturing and logistics
- No requirement to identify personalized tumor antigens
PERSONALIZED
- Induction of immune responses against personalized antigens
- Cross-presentation within tumor microenvironment by cDC1-like cancer cells
- Explores multiple mechanisms of action
- Targets tumor heterogeneity and bypasses tumor immune evasion